Endothermic reactions vs exothermic reactions worksheet answers – Unveiling the mysteries of chemical reactions, this discourse delves into the captivating realm of endothermic and exothermic reactions. As we embark on this intellectual journey, we unravel the intricate dynamics of energy flow, temperature shifts, and heat transfer, deciphering the fundamental differences that set these reactions apart.
Through a comprehensive exploration of their definitions, mechanisms, and practical applications, we illuminate the profound impact of these reactions in shaping our world. From everyday phenomena to industrial processes, endothermic and exothermic reactions play a pivotal role in countless aspects of our lives.
Endothermic and Exothermic Reactions: Endothermic Reactions Vs Exothermic Reactions Worksheet Answers
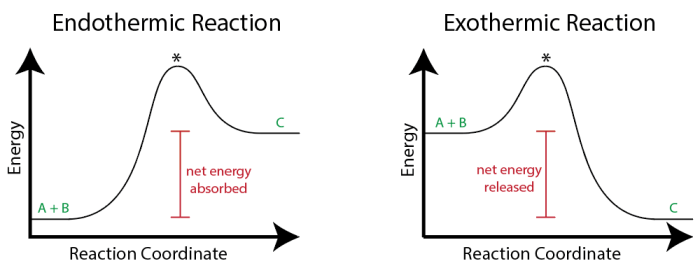
Chemical reactions can be classified as endothermic or exothermic based on the energy exchange that occurs during the reaction. Understanding the differences between these two types of reactions is essential in chemistry.
1. Define and Explain Endothermic Reactions
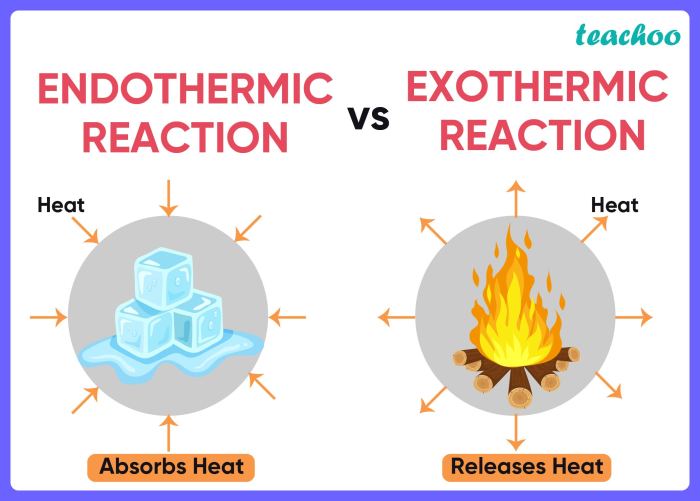
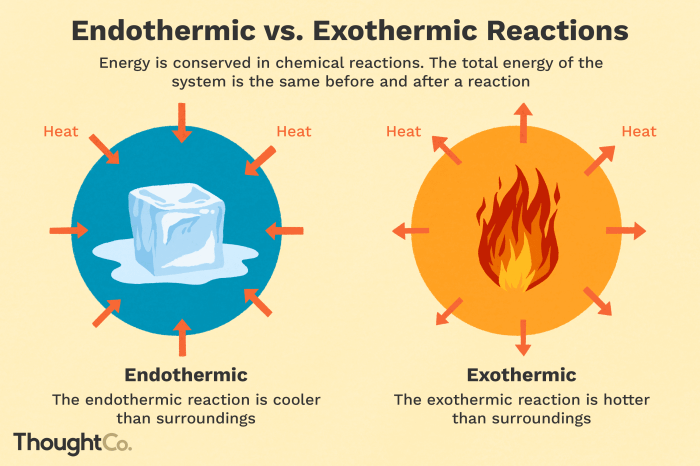
Endothermic reactions are chemical reactions that absorb energy from the surroundings in order to proceed. This energy is typically in the form of heat, which is transferred from the environment to the reactants. As a result, the temperature of the surroundings decreases during an endothermic reaction.
2. Define and Explain Exothermic Reactions
Exothermic reactions are chemical reactions that release energy into the surroundings as they proceed. This energy is typically in the form of heat, which is transferred from the products to the environment. As a result, the temperature of the surroundings increases during an exothermic reaction.
3. Comparison of Endothermic and Exothermic Reactions, Endothermic reactions vs exothermic reactions worksheet answers
The following table summarizes the key differences between endothermic and exothermic reactions:
| Characteristic | Endothermic Reactions | Exothermic Reactions |
|---|---|---|
| Energy Flow | Absorb energy from surroundings | Release energy into surroundings |
| Temperature Change | Temperature decreases | Temperature increases |
| Heat Transfer | Heat flows from surroundings to reactants | Heat flows from products to surroundings |
4. Applications of Endothermic and Exothermic Reactions
Endothermic reactions are used in a variety of applications, including:
- Refrigeration and air conditioning
- Melting ice and snow
- Cooking and baking
Exothermic reactions are used in a variety of applications, including:
- Fireworks and explosives
- Welding and cutting metals
- Production of cement and glass
Commonly Asked Questions
What is the key difference between endothermic and exothermic reactions?
Endothermic reactions absorb energy from their surroundings, resulting in a decrease in temperature, while exothermic reactions release energy into their surroundings, leading to an increase in temperature.
Provide an example of an endothermic reaction.
Dissolving ammonium chloride in water is an endothermic reaction, as it absorbs heat from the surroundings, causing the solution to become cooler.
What is a common application of exothermic reactions?
Exothermic reactions are commonly used in fireworks, as the release of energy creates the dazzling displays of light and sound.