Using the activity series provided which reactants will form products – In the realm of chemistry, the activity series serves as an invaluable tool for predicting the products of reactions. This article delves into the intricacies of the activity series, exploring its fundamental principles and demonstrating its practical applications. By understanding the reactivity trends of metals, we can gain insights into the spontaneity and outcomes of chemical reactions.
The activity series, a ranking of metals based on their reactivity, provides a systematic framework for predicting the formation of products. Metals higher in the series exhibit greater reactivity, while those lower in the series are less reactive. This reactivity gradient stems from the differences in their ionization energies and electron configurations.
Overview of the Activity Series
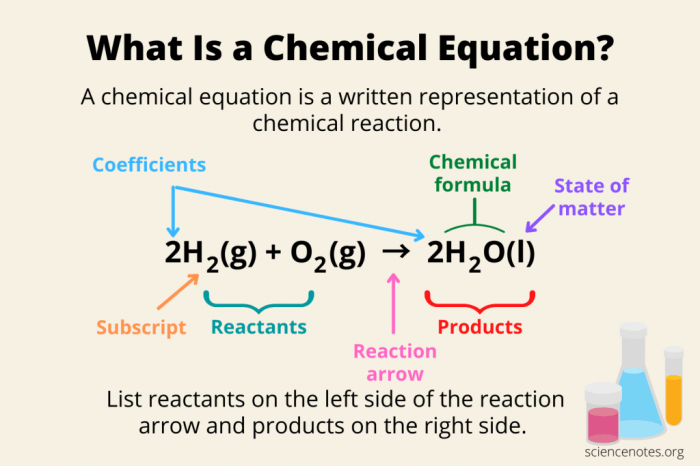
The activity series is a list of metals arranged in order of their reactivity. The more reactive a metal is, the higher it is on the list. Reactivity is a measure of how easily a metal can lose electrons and form positive ions.
The activity series can be used to predict the products of a reaction between two metals.
The factors that affect the reactivity of metals include their atomic number, their ionization energy, and their electron affinity. Atomic number is the number of protons in the nucleus of an atom. Ionization energy is the energy required to remove an electron from an atom.
Electron affinity is the energy released when an electron is added to an atom.
Using the Activity Series to Predict Reactions: Using The Activity Series Provided Which Reactants Will Form Products
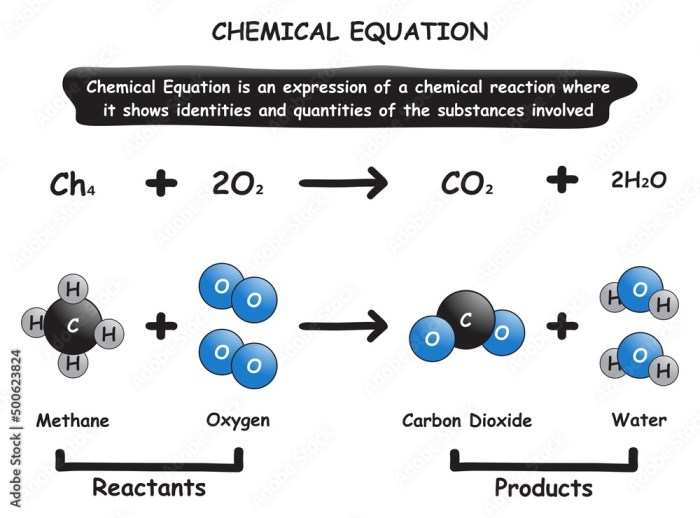
The activity series can be used to predict the products of a reaction between two metals by comparing the reactivity of the metals. The more reactive metal will lose electrons and form positive ions, while the less reactive metal will gain electrons and form negative ions.
The positive ions will then combine with the negative ions to form a compound.
For example, if iron and copper are placed in a solution of copper sulfate, the iron will react with the copper sulfate to form iron sulfate and copper. This is because iron is more reactive than copper, so it will lose electrons and form positive ions, while copper will gain electrons and form negative ions.
Limitations of the Activity Series, Using the activity series provided which reactants will form products
The activity series is not always accurate in predicting the products of a reaction. This is because there are other factors that can affect the reactivity of metals, such as the concentration of the reactants, the temperature of the reaction, and the presence of a catalyst.
For example, if iron and copper are placed in a solution of copper sulfate at a high temperature, the copper will react with the copper sulfate to form copper oxide and iron sulfate. This is because the high temperature increases the reactivity of the copper.
Applications of the Activity Series
The activity series has a number of practical applications. It can be used to predict the products of a reaction, to design corrosion-resistant materials, and to develop new electrochemical cells.
For example, the activity series can be used to predict the products of a reaction between a metal and an acid. The more reactive the metal, the more easily it will react with the acid. This information can be used to design corrosion-resistant materials, such as stainless steel, which is resistant to corrosion because it contains a high percentage of chromium, a metal that is less reactive than iron.
Clarifying Questions
What is the activity series?
The activity series is a ranking of metals based on their reactivity. Metals higher in the series are more reactive than those lower in the series.
How can the activity series be used to predict reaction products?
The activity series can be used to predict the products of a reaction by comparing the reactivity of the reactants. A more reactive metal will displace a less reactive metal from a compound.
What are the limitations of the activity series?
The activity series is not always accurate in predicting reaction products. Factors such as temperature, concentration, and the presence of catalysts can affect the outcome of a reaction.